CURRENT RESEARCH PROJECTS OF THE POLLMANN LAB - Plant cell walls
Plants are frequently challenged by unfavorable conditions in their ever-changing and often hostile environment. They are forced to respond and adjust to a multitude of different external cues and coordinate their growth and developmental program accordingly. To cope with problems arising from their sessile lifestyle, they have evolved complex solutions to ensure their survival. For example, plants have developed multiple layers of sophisticated surveillance mechanisms that recognize potentially dangerous pathogens and rapidly respond before those organisms have a chance to cause serious damage. These surveillance systems include basal resistance, also called innate immunity, and hypersensitive responses. Moreover, plants posses additional levels of elaborate chemical defense that rely on the production or liberation of toxic primary and/or secondary metabolites or proteins and enzymes that display broad anti-microbial activity or specifically inhibit pathogen and pest enzymes to ultimately reduce their function.
The cell wall is a major line of defense against fungal and bacterial pathogens
Apart from these complex defense systems, plants are characterized by a number of structural features that provide protection for the plant body. In the plant kingdom, structural defense already begins at the cellular level. Plant cells are surrounded by cell walls that represent a major line of defense against fungal and bacterial pathogens. On average, plant cell walls contain 75% of sugar. Most of this sugar is deposited in form of long polysaccharide chains, termed cellulose. Cellulose consists of several hundred to over 10,000 β(1→4) linked D-glucose units that prevade, joint to fibrils, the cell wall. Besides cellulose, plant cell walls contain several other components, such as hemicellulose, pectin, and lignin. Especially the latter serves as a structural barrier that helps limit pathogen invasion and infection as it acts as a kind of a cellular concrete that links the various biopolymers and molecules in a very rigid manner.
In addition, the outermost protective cell layer of plants, the epidermis, constitutes a protective tissue system of leaves, floral parts, fruits, seeds, stems, and roots that serves as the first line of defense against invading pathogens. Epidermal cells of aerial plant parts are often covered in a waxy cuticle that not only prevents water loss from the plant, but also prevents microbial pathogens from coming into direct contact with epidermal cells and thereby limits infection. Some plants also posses specialized outgrowths of the epidermis, i.e. trichomes and prickles, that prevent insect eggs from reaching the epidermis and protect plants from grazing vertebrates. Others plants show different modified plant organs, such as branches (thorns) and leaves or parts of leaves (spines), which serve the same purpose.
In a related project, we observed that constitutive over-expression of two YUCCA genes, YUC8 and YUC9, led to aberrant secondary growth of the stem. In some cases the secondary growth was so pronounced that the stem was no longer able to follow the stronger growth and cracked open from the bottom to the top (Fig. 1). With respect to these findings, we thus hypothesized that increased disposal of stabilizing biopolymers, e.g. lignin and embedded cellulosic compounds, in the secondary cell walls is likely to be the causal link for the abnormal phenotype of the over-expressor lines relative to the wild type.
Figure 1. 35S-diven over-expression of both YUCCA8 and YUCCA9 confer an aberrant stem phenotype
When staining YUC8ox and YUC9ox plants for lignin contents, we were indeed able to confirm our hypothesis (Fig. 2). Apart from a significantly increased diameter of the stem, the over-expressor plants (right) exhibited a much stronger staining after phloroglucinol/HCl treatment, indicating higher levels of lignin in those lines relative to wild-type Arabidopsis (left).
Figure 2. Phloroglucinol/HCl staining of wild-type and YUCCA8 and YUCCA9 over-expressing plants. The over-expressor plants show more pronounced staining for lignin
Both plant development and plant stress responses can be regulated by the mutual interoperability and congruence of plant hormones; a concept that is widely accepted. Various examples for the crosstalk between phytohormones and explanations of the underlying molecular bases can be found in the literature (Hoffmann et al. 2011; Kazan and Manners 2008, 2011). For example, ethylene signaling is assumed to contribute to radial (horizontal) growth by modulating vascular cell division (Etchells et al. 2012). The stimulation of ethylene emission from plant tissues by exogenously applied as well as endogenously produced auxin is a well-established phenomenon (Abeles et al. 1964; Sitbon et al. 1999). Ethylene, for its part, also affects auxin biosynthesis and transport-dependent local auxin distribution (Ruzicka et al. 2007). Remarkably, recent findings significantly augmented the current insight into this intimate relationship between auxin and ethylene biosynthesis. Auxin and ethylene production are metabolically linked by a pyridoxal-phosphate-dependent aminotransferase, REVERSAL OF sav3 PHENOTYPE (VAS1), that catalyzes the transamination of IPA to L-tryptophan and 2-oxo-4-methylthiobutyric acid, specifically using methionine as the amino donor (Zheng et al. 2013). Overall, it seems as if there is a circle of mutual activation and a tight metabolic link between the two plant hormonal pathways. Most relevant, however, was the discovery that overproduction of IAA in transgenic plants induces the concomitant overproduction of ethylene (Sitbon et al. 1999).
Endogenous overproduction of IAA induces the concomitant biosynthesis of ethylene
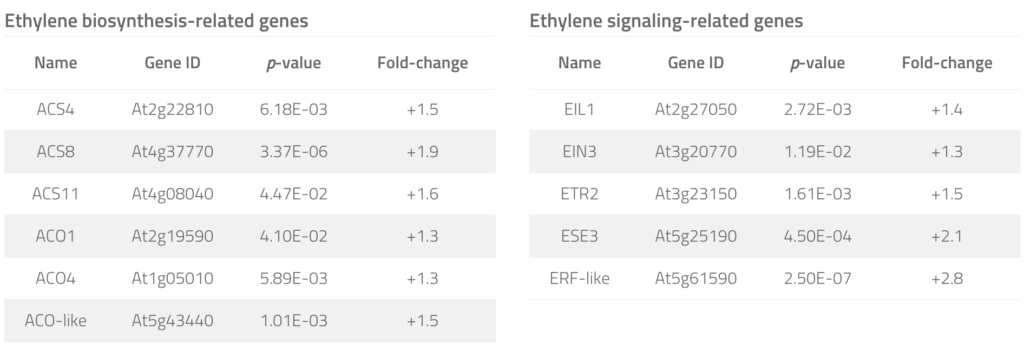
To examine whether YUC8– and YUC9-mediated IAA overproduction also affects ethylene biosynthesis, we tested the primary root growth inhibition by an ethylene biosynthesis inhibitor, 2-aminoisobutyric acid (AIB), in wild type and YUC8ox and YUC9ox lines. Although higher concentrations of AIB ultimately suppressed primary root growth in wild-type Arabidopsis as well as YUC8ox and YUC9ox, the two overexpression lines clearly showed hyposensitivity towards AIB, pointing towards an increased resistance that is mediated by the stimulated formation of ethylene. This result was confirmed by quantitative transcript analyses, which highlighted transcript accumulation of a number of ethylene biosynthesis- and signaling-related genes in YUC9ox (Tab. 1). Hence, we conclude that YUC8 and YUC9 overexpression-mediated overproduction of IAA, in turn, triggers the induction of ethylene production and signaling, which in combination stimulates secondary growth and deposition of lignin into the cell walls.
Table 1. Ethylene biosynthesis and signaling components significantly affected in their expression in the YUC9 over-expression line
At present, our lab is interested in the elucidation of the molecular mechanism(s) through which plant stress responses are linked with auxin/ethylene crosstalk and lignin formation. Very much like in our other projects, we currently undertake a transcriptomics approach along with mass spec-based analytic experiments to tackle our objectives.