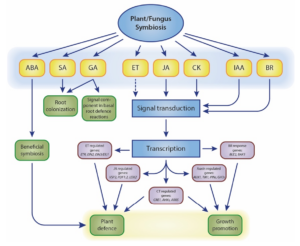
Our work primarily focuses on root-colonising fungal endophytes. Symbiosis between non-mycorrhizal fungi and host plants occurs in two stages: a brief biotrophic phase followed by a saprophytic phase upon symbiosis establishment (Zuccaro et al. 2011; Lahrmann et al. 2013). Initially, the fungus contacts the root, causing rapid pH changes in the rhizosphere (Lanza et al. 2019). The host plant responds by secreting specific symbiotic proteins (Thürich et al. 2018). Concurrently, plant innate immunity is suppressed by manipulating multiple hormone signalling pathways to overcome plant defences and ensure compatibility (Johnson et al. 2011; Pérez-Alonso et al. 2022). Levels of abscisic acid (ABA), salicylic acid (SA), jasmonic acid (JA), and jasmonoyl-L-isoleucine significantly increase during the initial interaction stages. Although the exact mechanisms of plant hormone crosstalk in symbiotic interactions remain debated (Fig. 1), the essential role of plant hormones in coordinating these interactions is widely acknowledged.
Figure 1. Schematic overview of plant hormone-related processes influenced by fungal symbionts. Dashed lines represent undefined roles. Crosstalk between plant hormones is not indicated. The model is adapted from (Xu et al. 2018).
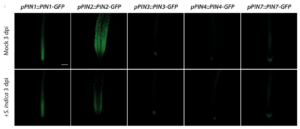
Our previous work demonstrated the critical contribution of fungus-dependent readjustment of local auxin maxima in plant root tips through a precise and subtle repression of the auxin exporter gene PIN2 (Fig. 2). The resulting auxin maxima in the tips were shown to remain under regulation by the local induction of the GRETCHEN HAGEN 3 genes GH3.5 and GH3.17 (González Ortega-Villaizán et al. 2024).
Figure 2. Qualitative and quantitative analysis of PIN transporter responses to an infection with S. indica. Confocal laser scanning microscopy images of primary root tips of mock‐ and S. indica‐infected PIN‐GFP promoter‐reporter plants at 3 dpi. Scale bar = 100 µm.
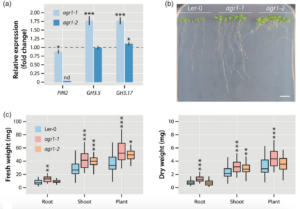
Through the utilisation of the partial PIN2 mutants agr1-1 and agr1-2 (Chen et al. 1998; Utsuno et al. 1998), illustrated in Fig. 3, we were also able to demonstrate that a significant portion of the growth-promoting effect of Serendipita indica on Arabidopsis thaliana is dependent on this mechanism. The weaker allele, agr1-1, was observed to induce the expression of GH3.5 and GH3.17 independently of S. indica and exhibits increased biomass compared to wild-type Arabidopsis plants.
Figure 3. Transcriptional and physiological effects of a partial loss of PIN2. (a) Quantification of PIN2, GH3.5, and GH3.17 expression in the mutants agr1‐1 and agr1‐2 relative to the Ler‐0 wild type (dashed line) by qRT‐PCR. The bars show means of n = 3 independent measurements. Asterisks mark the experiments with significantly altered gene expressions. Student’s t test: *p ≤ 0.05, ***p ≤ 0.001. (b) Phenotype of the used Arabidopsis genotypes, Ler‐0, agr1‐1, and agr1‐2 grown for 10 days on vertical 0.5 × MS plates. Scale bar = 1 cm. (c) Fresh and dry weight measurements of the tested Arabidopsis genotypes. The box plots show the median, quartiles, and extremes of the compared data sets (n = 32). Asterisks indicate significant differences between the Ler‐0 control and the corresponding agr1‐1 and agr1‐2 samples. Student’s t test: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Along with our previous work on the role of the Ca2+ sensor CBL7 (Pérez-Alonso et al. 2022), this study substantially improved the current understanding on the sophisticated regulatory mechanisms involved in the symbiosis between S. indica and A. thaliana. A possible biotechnological exploitation of this finding is currently being investigated.
Fungal endophytes reprogram a complex regulatory network involving plant hormones and other secondary messengers to promote plant growth and improve stress tolerance.
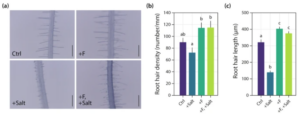
Recent research from our lab has also elucidated a molecular mechanism by which the fungus Fusarium sp. strain K-23, isolated from the Himalaya, confers increased salt stress tolerance to Arabidopsis (Onejeme et al. 2024). As depicted in Fig. 4, this mechanism involves the promotion and maintenance of root hair growth. Reverse genetics experiments utilising the root hairless rsl2 rsl4 double mutant further elucidated that the capacity for root hair growth is crucial to the observed salt stress tolerance enhancement in wild-type plants.
Figure 4. K-23-promoted root hair elongation. (a) Representative images of root hairs from Arabidopsis control seedlings (Ctrl) grown for 7 days on 0.5 × MS and another 7 days on PNM minimal medium, similarly grown seedlings but with 50 mM NaCl (+ Salt) in the PNM medium, and seedlings infected with K-23 in the absence (+ F) and presence of 50 mM NaCl (+ F, + Salt) at 7 dpi. Scalebar: 400 μm. (b) Quantification of root hair density and © root hair length. Data are means ± SE (n = 10 roots). One-way ANOVA with a post hoc Tukey–Kramer test was used. Different letters indicate significant differences between means (p ≤ 0.05).
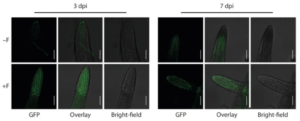
Furthermore, our transcriptomics experiments resulted in the identification of the NAC transcription factor JUNGBRUNNEN 1 (JUB1) as a determinant involved in maintaining root hair growth under salt stress conditions. JUB1 has previously been reported to regulate gibberellic acid (GA) and brassinosteroid (BR) levels in plants (Shahnejat-Bushehri et al. 2016). Through the utilisation of plant hormone signalling reporter lines and advanced confocal laser scanning microscopy (Fig. 5), we were able to confirm the stabilisation of the DELLA co-repressor protein RGA, which is known to promote root hair growth (Jiang et al. 2007).
Figure 5. Infection of Arabidopsis roots with Fusarium sp. strain K-23 promotes DELLA stability in roots. GFP-fluorescence and bright-field image as well as the overlay of the two images showing lateral root tips of mock- (−F) and K-23-infected (+ F) RGAp::GFP-RGA GA-signalling reporter plants at 3 and 7 dpi. Scale bars = 500 µm.
Our investigation yielded substantial evidence for previously undescribed molecular mechanisms triggered by fungal plant symbionts that enhance abiotic stress tolerance in plants. We identified several regulatory circuits that are crucial for governing the fungus-triggered processes. Finally, our research and the described plant-fungus systems may also serve as valuable molecular tools to further elucidate plant developmental processes under various abiotic stress conditions.